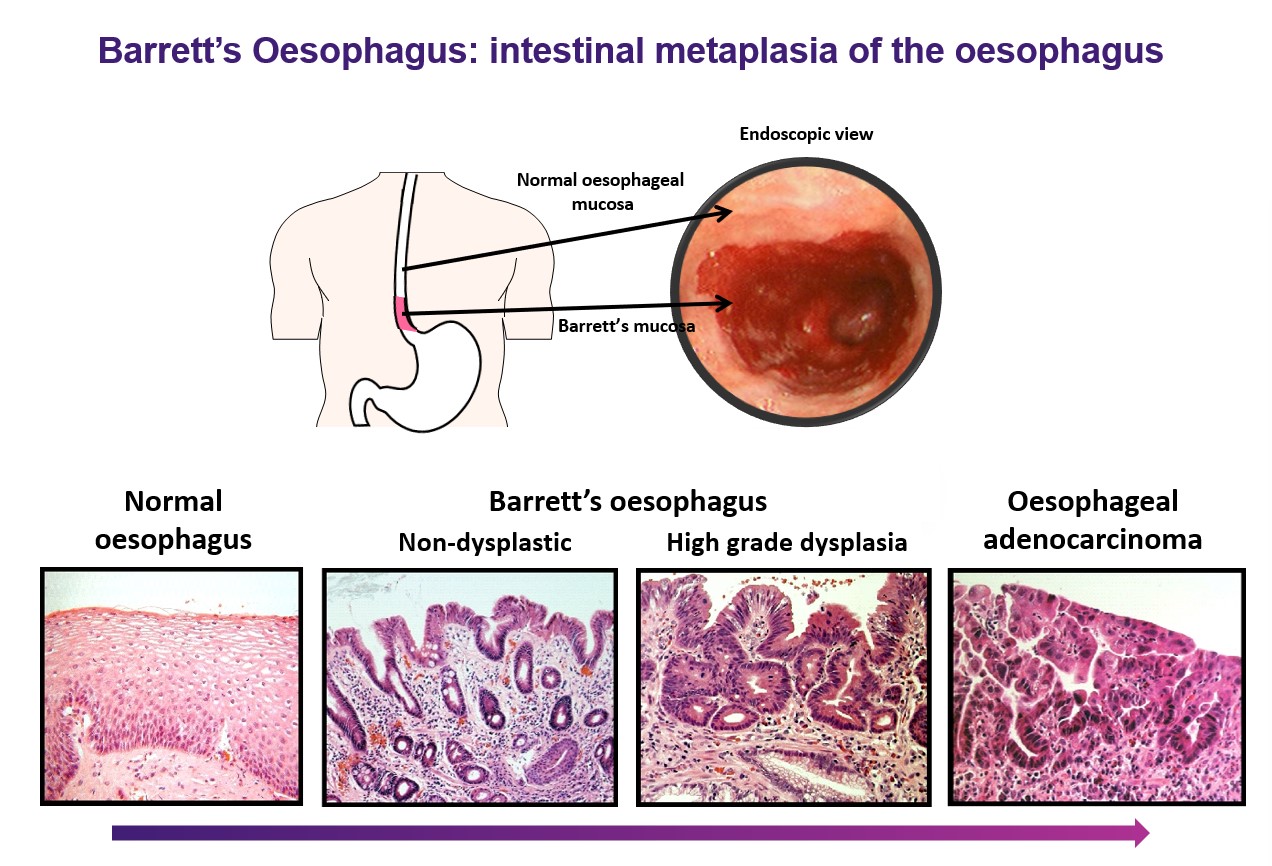
Barrett’s oesophagus is a metaplasia, acquired as a consequence of chronic acid and bile reflux, where the normal squamous epithelium is replaced by an intestinal-type columnar epithelium. Clinically, Barrett’s oesophagus is important as it is the only known precursor for oesophageal adenocarcinoma. Up to 2% of adults in western populations are thought to have Barrett’s oesophagus, which carries a ~30-fold greater risk for developing oesophageal adenocarcinoma compared to the general population.
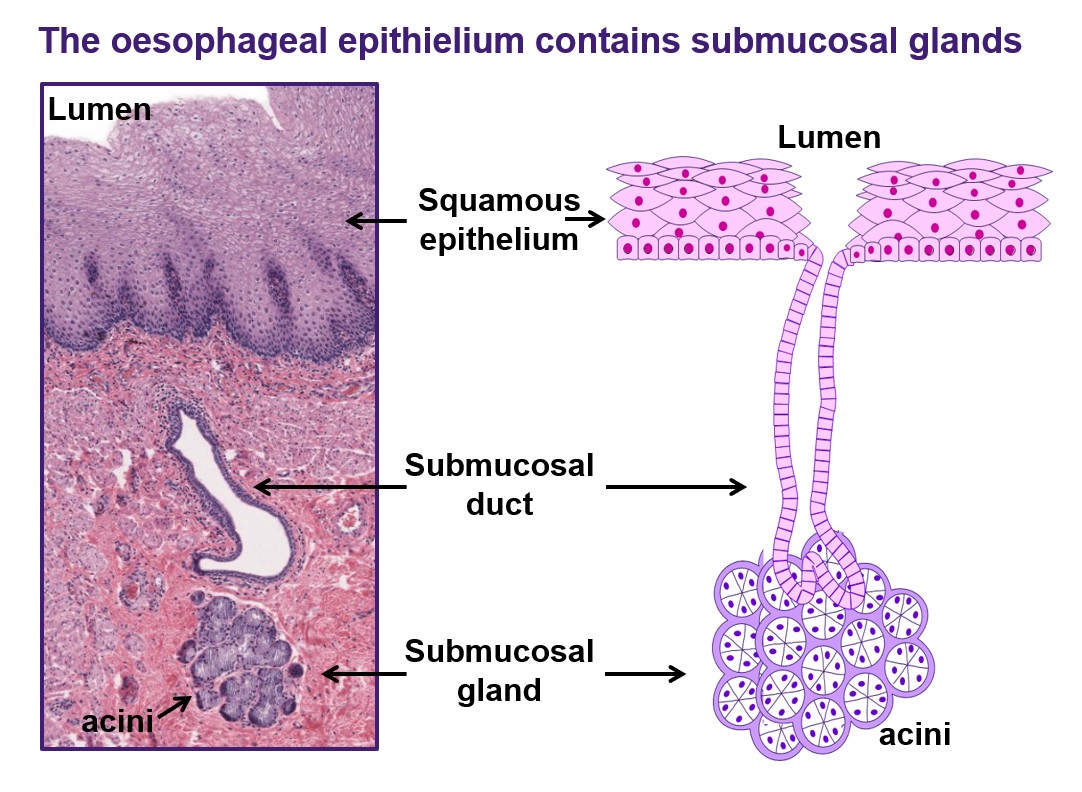
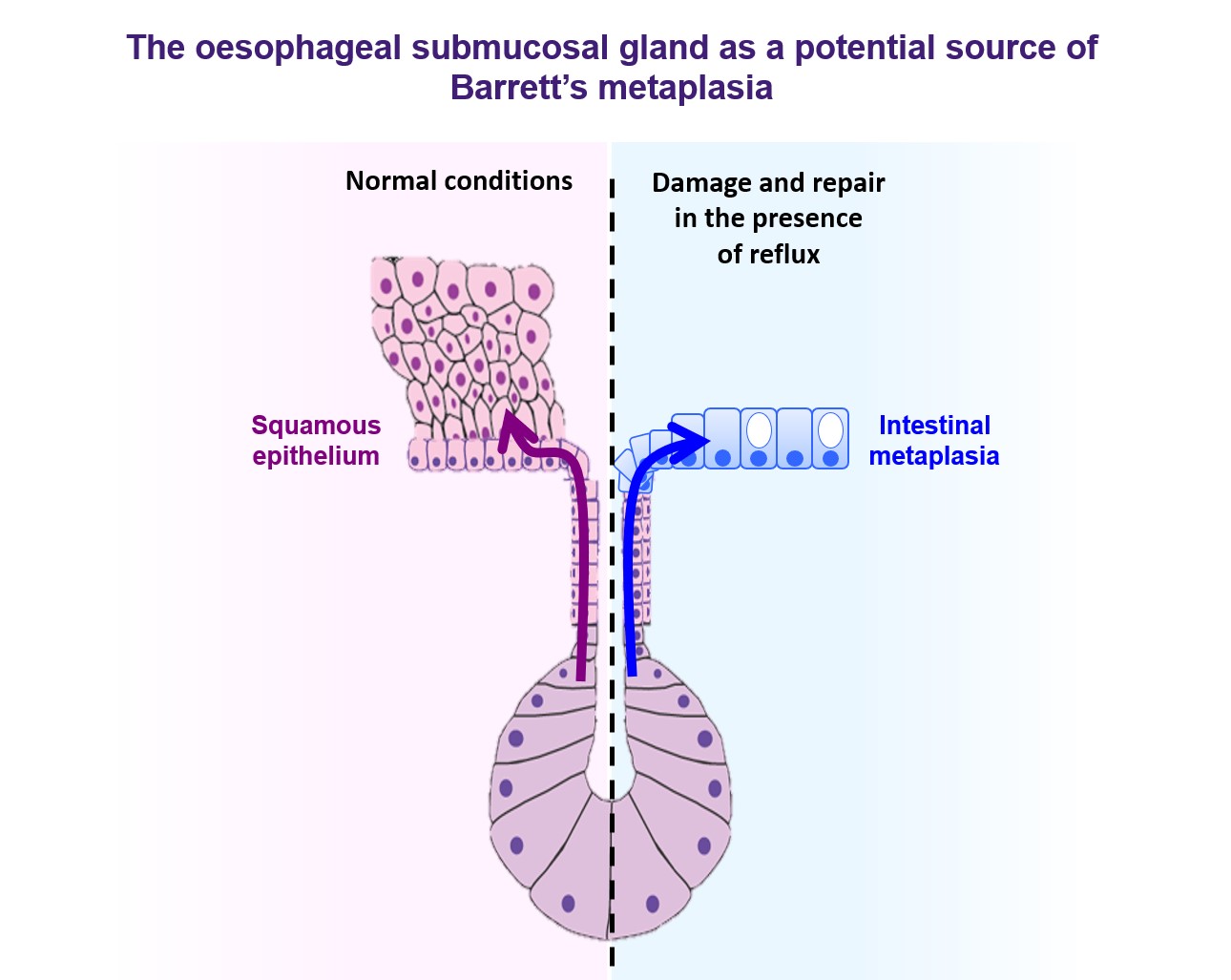
Currently, efforts to develop effective strategies to predict or prevent progression, or even reverse Barrett’s oesophagus, are hampered by a general lack of understanding of the fundamental biology underlying this important pre-cancerous condition. Indeed, we do not even know where the intestinal-type columnar cells that characterise Barrett’s metaplasia actually come from. This crucial gap in our knowledge is preventing us from making significant inroads into reducing the incidence of, and mortality from, oesophageal adenocarcinoma.
The Wayne Phillips lab is focused on understanding the cellular and molecular mechanisms underlying development of Barrett’s oesophagus and its progression to adenocarcinoma. We are using our unique collection of innovative preclinical models to provide critical new insights into the factors driving the metaplasia-dysplasia-adenocarcinoma sequence in the oesophagus and identify novel targets, new treatment strategies, and effective prognostic and predictive biomarkers, in order to deliver new strategies to prevent, treat, and/or guide clinical decisions and thus improve outcomes for patients with oesophageal cancer.