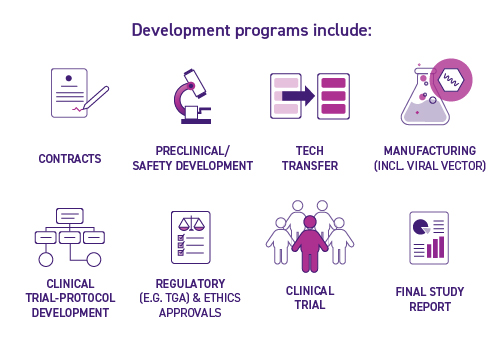
One of the key goals of the Centre of Excellence in Cellular Immunotherapy is to facilitate the increase in cellular immunotherapy clinical trial activity. As such, the Centre of Excellence in Cellular Immunotherapy’s Pilot Clinical Trial Development Program was established as an accelerator program to facilitate early phase investigator initiated and collaborative clinical trials. We do this by providing support and expertise to co-develop and manufacture innovative, cell-based immunotherapy concepts with our partners for rapid translation into pilot clinical trials with potential for commercial outcomes.
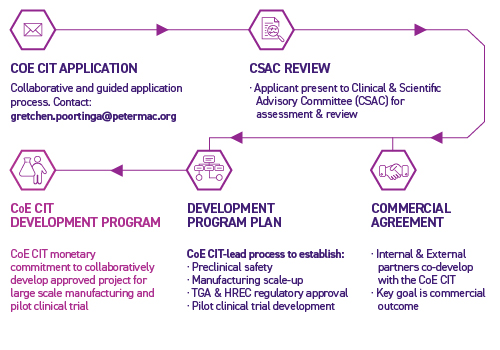
Centre of Excellence in Cellular Immunotherapy Pilot Clinical Trial Development Program Application Process
Acceptance into the Centre of Excellence in Cellular Immunotherapy Pilot Clinical Trial Development Program is based on an application process. Our Clinical and Scientific Advisory Committee (CSAC) will review your application and, if successful, the Centre of Excellence in Cellular Immunotherapy team, in collaboration with our legal and commercial teams, will negotiate and execute a commercial agreement.
Centre of Excellence in Cellular Immunotherapy application
Download the Centre of Excellence in Cellular Immunotherapy application
Frequently asked questions about the Pilot Clinical Trial Development Program
Contact
For more information contact
