News
08 June 2022
We provide a precinct wide Medical Oncology service

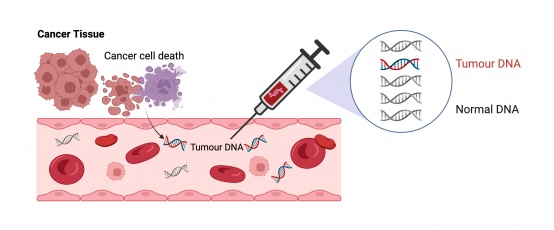
Researchers have developed a new blood test that could revolutionise how chemotherapy is used for colon cancer, by identifying the patients that need chemo and those that can be safely spared treatment. More than 450 patients and over 20 hospitals from across Australia were involved in the DYNAMIC trial, which investigated the blood test as a promising aid to cancer treatment decision-making. The study – co-led by WEHI and the Johns Hopkins Kimmel Cancer Centre in the US, and with the Peter MacCallum Cancer Centre as the lead clinical site – found the test could accurately predict which patients would benefit from chemotherapy after their cancer is surgically removed. Stage II colon cancer is defined as a cancer that has grown through the wall of the colon, but does not extend to the lymph nodes or other organs. Nearly 4,000 Australians are diagnosed with it each year. While most patients with stage II colon cancer are cured after surgery to remove the cancer from the bowel, the cancer will recur in around 20 per cent of patients. Chemotherapy is currently offered to all stage II colon cancer patients despite a majority not needing it. The goal of chemotherapy when given after surgery is to eradicate micrometastases – cancer cells that have travelled from the original cancer in the bowel through the bloodstream to deposit in another site, such as the liver. These deposits of cancer cells are miniscule and are not seen at surgery or on radiologic images during the initial stages. However, they will gradually increase in size and become large enough to be seen on a standard CT scan if they are not treated with chemotherapy. Associate Professor Jeanne Tie – a medical oncologist at Peter Mac and a clinician scientist at WEHI – was part of the team developing the blood test. The test can detect these invisible cells that release tiny amounts of circulating tumour DNA (ctDNA) – genetic material shed from tumours into the bloodstream, enabling researchers to identify which patients should be offered chemotherapy based on whether micrometastases has been detected. "Our trial has conclusively shown how the ctDNA blood test can be used to direct post-surgical therapy in stage II colon cancer and substantially reduce the number of patients treated with chemotherapy, without impacting the risk of cancer relapse," Associate Professor Tie said. "We found that when a patient's blood test does not reveal ctDNA after colon surgery, the likelihood of micrometastases is very low and chemotherapy can be avoided as there are no tumour fragments left to kill." The findings, published in The New England Journal of Medicine over the weekend, are globally significant as previously there was no way of determining whether all the cancer had been removed through colon surgery. “While chemotherapy can be essential and lifesaving, many patients are receiving the treatment and its associated toxicities without any benefit,” Associate Professor Tie said. “This ctDNA blood test could be used to spare around 600 Australians and over 100,000 people worldwide from unnecessary chemo treatments each year.” Lead image: Sangharsh Lohakare, Unsplash