The immune system consists of many cells and organs. They almost always protect the body from infection and cancer. An important part of the immune system is T cells. T cells can hunt down and destroy abnormal cells, including some cancer cells. Sometimes cancer cells find ways to evade the immune system. When this happens, we need to retrain the immune system to recognise and attack cancer cells. CAR T-cell therapy is one way that we can train and strengthen the immune system to attack some forms of cancer.
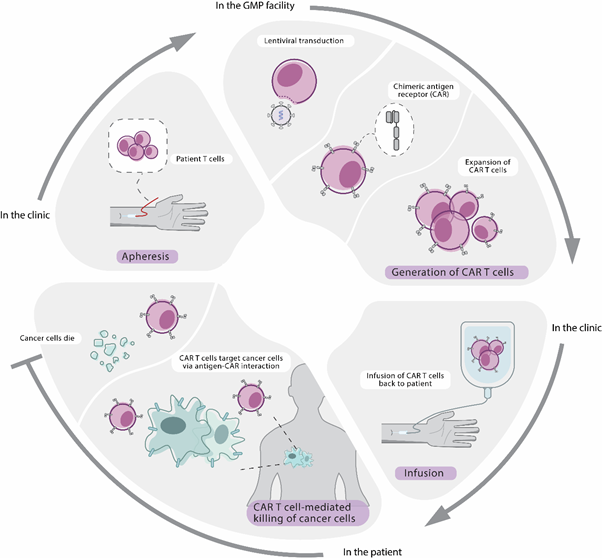
We first collect a small part of your T cells from your blood. We then re-engineer the T cells in a special laboratory. This lets them carry special structures on their surface. We call these structures chimeric antigen receptors (CARs).
We then add the T cells back into you as CAR T cells, where they will multiply. These engineered receptors may help the T cells attack cancer cells throughout your body.
The following video supplies a more in-depth look into how this works:
You can also view the longer version of What is CAR T-cell therapy (animation by Maja Divjak)
Approved Therapies
We have approval to use CAR T-cell therapy in Australia for the following diseases:
- B-cell acute lymphoblastic leukaemia (ALL)
- Adult diffuse large B-cell lymphoma (DLBCL)
- Mantle cell lymphoma (MCL)
- Multiple Myeloma
CAR T-cell treatments for solid cancers are not approved in Australia. We are doing ongoing research to develop new CAR-based treatments for solid cancers. We also are running clinical trials for some solid cancers.
CAR T-cell therapy is different from allogeneic (from a donor) stem cell transplant. Nor does it replace allogenic stem cell transplants. Find out more information on allogeneic transplants.
Access to Cell therapy
Talk with your haematologist or oncologist. They can find out if CAR T-cell therapy is a suitable treatment for you.
Regulatory approval
Therapeutic Goods Administration (TGA) approves therapies in Australia. It has approved us to use the following CAR T-cell therapies:
Kymriah® (tisagenlecleucel)
We have approval to use this for paediatric and adolescent acute lymphoblastic leukaemia (ALL) (post-transplant) or in second or later relapse. This is also approved for adult relapsed or refractory diffuse large B cell lymphoma (DLBCL) after two or more lines of systemic therapy.
Yescarta® (Axicabtagene ciloleucel)
We have approval to use this for adult relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy.
Tecartus® (brexucabtagene autoleucel)
We have approval to use this for adult relapsed or refractory mantle cell lymphoma (MCL), who have received two or more lines of therapy. This includes a BTK inhibitor (unless ineligible or intolerant to treatment with a BTK inhibitor).
CARVYKTI® (Ciltacabtagene autoleucel)
We have approval to use this for adult patients with relapsed or refractory multiple myeloma who have received at least three prior lines of therapy.
Funding approval
The Australian Government funds all CAR T-cell therapy for the following situations:
- Children and young people up to 25 years of age. They must have B-cell precursor acute lymphoblastic leukaemia (ALL) in:
- Relapse post-transplant
- Second or later relapse Adults with relapsed or refractory diffuse large B cell lymphoma (DLBCL) after two or more lines of systemic therapy
The Medical Services Advisory Committee’s recommendations inform these funding schemes.
The Australian government does not subsidise treatment with other approved CAR T-cell therapies or for other indications.
Clinical trials
We have practiced cellular immunotherapies for over 10 years. We performed Australia’s very first CAR (Chimeric Antigen Receptor)-T cell clinical trial. We continue to look for new uses of CAR T-cell therapy in clinical trials. We are looking to enrol specific patients into clinical trials in:
- Leukaemia
- Lymphoma
- Myeloma
- Solid cancers
Joining a CAR T-cell therapy clinical trial
- Check the availability of clinical trial with us by emailing
This email address is being protected from spambots. You need JavaScript enabled to view it. - You should contact your haematologist or oncologist to discuss your suitability
- Have your health professional organise the appropriate referrals. They should then forward these to us
Please email
